For US Healthcare Professionals


ESR1m are found in ~40% of mBC tumors at progression on prior ET2

Tumors acquire ESR1m directly
under pressure from prior ET, primarily AI4

Unlike PIK3CA/AKT/PTEN mutations that may not have been treated since mBC diagnosis, ESR1m are acquired at progression2,5-9
Addressing ESR1m presents an immediately actionable opportunity to treat the evolving tumor biology1,2
Test for ESR1m to treat what may be driving their disease progression in 2L1-4
2L, 2nd Line; AI, aromatase inhibitor; AKT, protein kinase B; ER+, estrogen receptor-positive; ESR1, estrogen receptor 1; ESR1m, estrogen receptor 1 mutation; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; mBC, metastatic breast cancer; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; PTEN, phosphatase and tensin homolog.

-
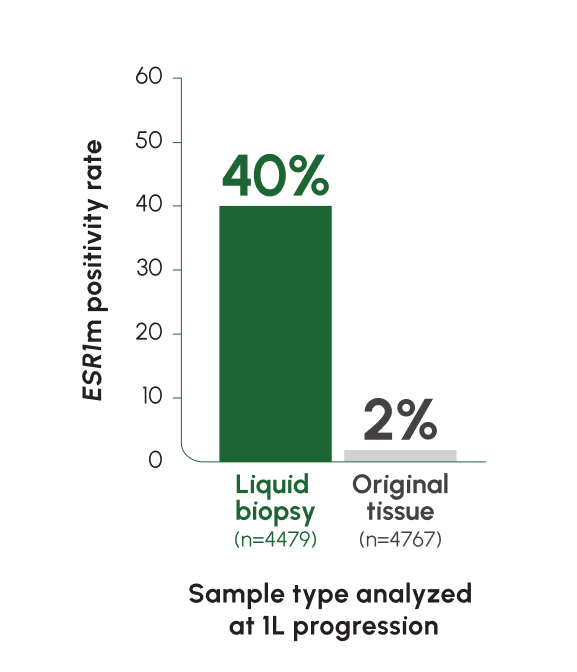
ESR1 mutations are detected at a rate of 5% at mBC diagnosis, with an increase of up to ~40% following progression on ET2,12
-
Tumors from different sites of metastases may have different genetic compositions. Liquid biopsy captures ctDNA from multiple locations and reflects tumor heterogeneity more accurately than tissue biopsy13
NCCN GUIDELINES® RECOMMENDATION
NCCN Guidelines® recommend evaluating ESR1 mutation status using next-generation sequencing or PCR, preferably with blood samples. NCCN Guidelines does not recommend testing with primary archived tissue given the acquired nature of ESR1 mutations.14,15
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. Refer to NCCN Guidelines for full recommendations.
|
|
|
|
|
|||
At every
|
Test for ESR1m
|
If ESR1m
|
Prescribe
|
-
Test for ESR1m at each progression if not detected previously, as ESR1 mutations may emerge upon repeated exposure to endocrine therapy1,16,17
-
Select patients for treatment with ORSERDU based on the presence of an ESR1 mutation in plasma specimen (ctDNA) using an FDA-approved test1
-
ctDNA can provide a more complete biomarker profile through liquid biopsy13,18
-
Due to their emergent nature, ESR1 mutations are rarely found in primary tumors—testing archival tissue is considered inadequate19
Test for ESR1 mutations. Treat with ORSERDU.
1L, 1st Line; ctDNA, circulating tumor DNA; ER+, estrogen receptor-positive; ESR1, estrogen receptor 1; ESR1m, estrogen receptor 1 mutation; ET, endocrine therapy; HER2-, human epidermal growth factor receptor 2-negative; mBC, metastatic breast cancer; NCCN, National Comprehensive Cancer Network® (NCCN®); PCR, polymerase chain reaction.
Learn more about the EMERALD trial
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
-
Dyslipidemia: Hypercholesterolemia and hypertriglyceridemia occurred in patients taking ORSERDU at an incidence of 30% and 27%, respectively. The incidence of Grade 3 and 4 hypercholesterolemia and hypertriglyceridemia were 0.9% and 2.2%, respectively. Monitor lipid profile prior to starting and periodically while taking ORSERDU.
-
Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, ORSERDU can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the last dose.
Adverse Reactions
-
Serious adverse reactions occurred in 12% of patients who received ORSERDU. Serious adverse reactions in >1% of patients who received ORSERDU were musculoskeletal pain (1.7%) and nausea (1.3%). Fatal adverse reactions occurred in 1.7% of patients who received ORSERDU, including cardiac arrest, septic shock, diverticulitis, and unknown cause (one patient each).
-
The most common adverse reactions (≥10%), including laboratory abnormalities, of ORSERDU were musculoskeletal pain (41%), nausea (35%), increased cholesterol (30%), increased AST (29%), increased triglycerides (27%), fatigue (26%), decreased hemoglobin (26%), vomiting (19%), increased ALT (17%), decreased sodium (16%), increased creatinine (16%), decreased appetite (15%), diarrhea (13%), headache (12%), constipation (12%), abdominal pain (11%), hot flush (11%), and dyspepsia (10%).
Drug Interactions
-
Concomitant use with CYP3A4 inducers and/or inhibitors: Avoid concomitant use of strong or moderate CYP3A4 inhibitors with ORSERDU. Avoid concomitant use of strong or moderate CYP3A4 inducers with ORSERDU.
Use in Specific Populations
-
Lactation: Advise lactating women to not breastfeed during treatment with ORSERDU and for 1 week after the last dose.
-
Hepatic Impairment: Avoid use of ORSERDU in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of ORSERDU in patients with moderate hepatic impairment (Child-Pugh B).
The safety and effectiveness of ORSERDU in pediatric patients have not been established.
ORSERDU is available as 345 mg tablets and 86 mg tablets.
INDICATION
ORSERDU (elacestrant) is indicated for the treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. ORSERDU [prescribing information]. New York, NY: Stemline Therapeutics, Inc., a Menarini Group Company, 2023. 2. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23(1):85. 3. Zhang K, Hong R, Xu F, et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res. 2018;10:2573-2580. 4. Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. 2019;125(21):3714-3728. 5. Mankoo PK, Sukumar S, Karchin R. PIK3CA somatic mutations in breast cancer: mechanistic insights from Langevin dynamics simulations. Proteins. 2009;75(2):499-508. 6. Casaubon JT, Kashyap S, Regan JP. BRCA1 and BRCA2 Mutations. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. 7. Arthur LM, Turnbull AK, Renshaw L, et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat. 2014;147(1):211-219. 8. Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388(22):2058-2070. 9. Clatot F, Perdrix A, Beaussire L, et al. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res. 2020;22(1):56. 10. Data on file. Stemline Therapeutics, Inc., a Menarini Group Company. 11. Bhave MA, Quintanilha JCF, Tukachinsky H, et al. Comprehensive genomic profiling of ESR1, PIK3CA, AKT1, and PTEN in HR(+)HER2(−) metastatic breast cancer: prevalence along treatment course and predictive value for endocrine therapy resistance in real‑world practice. Breast Cancer Res Treat. 2024;207(3):599-609. 12. Allouchery V, Beaussire L, Perdrix A, et al. Circulating ESR1 mutations at the end of aromatase inhibitor adjuvant treatment and after relapse in breast cancer patients. Breast Cancer Res. 2018;20(1):40. 13. Russano M, Napolitano A, Ribelli G, et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: the potentiality of blood samples. J Exp Clin Cancer Res. 2020;39(1):95. 14. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed April 17, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 15. Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw. 2023;21(6):594-608. 16. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):313ra182. 17. Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor–positive breast cancer. Clin Cancer Res. 2014;20(7):1757-1767. 18. Lee N, Park MJ, Song W, Jeon K, Jeong S. Currently applied molecular assays for identifying ESR1 mutations in patients with advanced breast cancer. Int J Mol Sci. 2020;21(22):8807. 19. Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579.
This site is intended only for US healthcare professionals. The products discussed in this site may have different product labeling in different countries. The information provided is for educational purposes only.
ORSERDU is a registered trademark of the Menarini Group.
© 2025 Stemline Therapeutics, Inc., a Menarini Group Company. All rights reserved. 12/25 MAT-US-ELA-00969-v3
|
IMPORTANT SAFETY INFORMATION
+
Warnings and Precautions
-
Dyslipidemia: Hypercholesterolemia and hypertriglyceridemia occurred in patients taking ORSERDU at an incidence of 30% and 27%, respectively. The incidence of Grade 3 and 4 hypercholesterolemia and hypertriglyceridemia were 0.9% and 2.2%, respectively. Monitor lipid profile prior to starting and periodically while taking ORSERDU.
-
Embryo-Fetal Toxicity: Based on findings in animals and its mechanism of action, ORSERDU can cause fetal harm when administered to a pregnant woman. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ORSERDU and for 1 week after the last dose.
Adverse Reactions
-
Serious adverse reactions occurred in 12% of patients who received ORSERDU. Serious adverse reactions in >1% of patients who received ORSERDU were musculoskeletal pain (1.7%) and nausea (1.3%). Fatal adverse reactions occurred in 1.7% of patients who received ORSERDU, including cardiac arrest, septic shock, diverticulitis, and unknown cause (one patient each).
-
The most common adverse reactions (≥10%), including laboratory abnormalities, of ORSERDU were musculoskeletal pain (41%), nausea (35%), increased cholesterol (30%), increased AST (29%), increased triglycerides (27%), fatigue (26%), decreased hemoglobin (26%), vomiting (19%), increased ALT (17%), decreased sodium (16%), increased creatinine (16%), decreased appetite (15%), diarrhea (13%), headache (12%), constipation (12%), abdominal pain (11%), hot flush (11%), and dyspepsia (10%).
Drug Interactions
-
Concomitant use with CYP3A4 inducers and/or inhibitors: Avoid concomitant use of strong or moderate CYP3A4 inhibitors with ORSERDU. Avoid concomitant use of strong or moderate CYP3A4 inducers with ORSERDU.
Use in Specific Populations
-
Lactation: Advise lactating women to not breastfeed during treatment with ORSERDU and for 1 week after the last dose.
-
Hepatic Impairment: Avoid use of ORSERDU in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of ORSERDU in patients with moderate hepatic impairment (Child-Pugh B).
The safety and effectiveness of ORSERDU in pediatric patients have not been established.
ORSERDU is available as 345 mg tablets and 86 mg tablets.
INDICATION
ORSERDU (elacestrant) is indicated for the treatment of postmenopausal women or adult men with estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy.
To report SUSPECTED ADVERSE REACTIONS, contact Stemline Therapeutics, Inc. at 1-877-332-7961 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. ORSERDU [prescribing information]. New York, NY: Stemline Therapeutics, Inc., a Menarini Group Company, 2023. 2. Brett JO, Spring LM, Bardia A, Wander SA. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23(1):85. 3. Zhang K, Hong R, Xu F, et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res. 2018;10:2573-2580. 4. Dustin D, Gu G, Fuqua SAW. ESR1 mutations in breast cancer. Cancer. 2019;125(21):3714-3728. 5. Mankoo PK, Sukumar S, Karchin R. PIK3CA somatic mutations in breast cancer: mechanistic insights from Langevin dynamics simulations. Proteins. 2009;75(2):499-508. 6. Casaubon JT, Kashyap S, Regan JP. BRCA1 and BRCA2 Mutations. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. 7. Arthur LM, Turnbull AK, Renshaw L, et al. Changes in PIK3CA mutation status are not associated with recurrence, metastatic disease or progression in endocrine-treated breast cancer. Breast Cancer Res Treat. 2014;147(1):211-219. 8. Turner NC, Oliveira M, Howell SJ, et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388(22):2058-2070. 9. Clatot F, Perdrix A, Beaussire L, et al. Risk of early progression according to circulating ESR1 mutation, CA-15.3 and cfDNA increases under first-line anti-aromatase treatment in metastatic breast cancer. Breast Cancer Res. 2020;22(1):56. 10. Data on file. Stemline Therapeutics, Inc., a Menarini Group Company. 11. Bhave MA, Quintanilha JCF, Tukachinsky H, et al. Comprehensive genomic profiling of ESR1, PIK3CA, AKT1, and PTEN in HR(+)HER2(−) metastatic breast cancer: prevalence along treatment course and predictive value for endocrine therapy resistance in real‑world practice. Breast Cancer Res Treat. 2024;207(3):599-609. 12. Allouchery V, Beaussire L, Perdrix A, et al. Circulating ESR1 mutations at the end of aromatase inhibitor adjuvant treatment and after relapse in breast cancer patients. Breast Cancer Res. 2018;20(1):40. 13. Russano M, Napolitano A, Ribelli G, et al. Liquid biopsy and tumor heterogeneity in metastatic solid tumors: the potentiality of blood samples. J Exp Clin Cancer Res. 2020;39(1):95. 14. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. Accessed April 17, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 15. Gradishar WJ, Moran MS, Abraham J, et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J Natl Compr Canc Netw. 2023;21(6):594-608. 16. Schiavon G, Hrebien S, Garcia-Murillas I, et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7(313):313ra182. 17. Jeselsohn R, Yelensky R, Buchwalter G, et al. Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor–positive breast cancer. Clin Cancer Res. 2014;20(7):1757-1767. 18. Lee N, Park MJ, Song W, Jeon K, Jeong S. Currently applied molecular assays for identifying ESR1 mutations in patients with advanced breast cancer. Int J Mol Sci. 2020;21(22):8807. 19. Spoerke JM, Gendreau S, Walter K, et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579.